
Empowering Global MS Research with the Neurostatus – EDSS:
the Neurostatus-EDSS Advantage
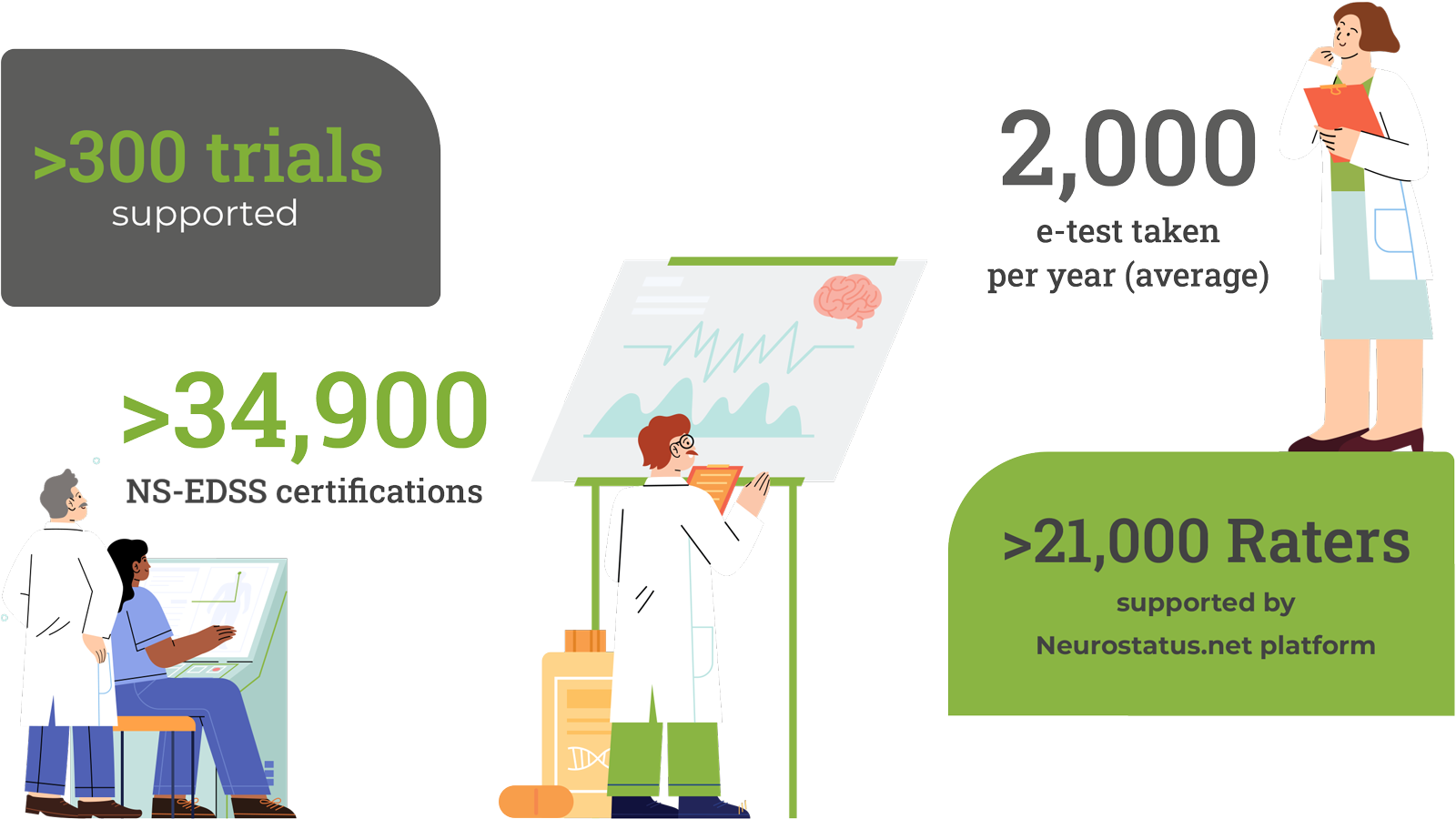
Improving data quality in Multiple Sclerosis Trials with Precision and Expertise
Neurostatus-UHB is the leading provider of expertise in the standardised neurological assessment of clinical symptoms in Multiple Sclerosis and related neuro-immunological diseases.
We support disease trials globally with our hallmark tool, the Neurostatus-Expanded Disability Status Scale (Neurostatus-EDSS).

What We Do

Two Facets of Excellence:
Paper & Digital
Neurostatus-UHB provides versatile solutions through both tangible and digital platforms. Whether it’s the familiarity of paper & pencil or the cutting-edge precision and reliability of our digital eEDSS eCOA system, we cater to all facets of clinical and academic research needs.
Improving Assessment Accuracy and Consistency with
the Neurostatus-eEDSS
Our digital innovation, the Neurostatus-eEDSS, elevates the original scale by introducing real-time, algorithm-based feedback and expert neurologist interactions. This dual-layer verification ensures unmatched consistency and reliability in assessment scoring, setting new standards in clinical trial data integrity.
Why We Do It

The Benchmark in MS Clinical Outcomes
The Neurostatus-EDSS approach offers a comprehensive, standardized method for assessing neurological impairments and disabilities in patients, critical for advancing therapeutic discoveries.
The Neurostatus-EDSS stands as one of the leading clinical outcome measures in the MS research domain. Integral to phase II and III MS trials, its precision and reliability have been central to the approval process of numerous Disease-Modifying Therapies (DMTs).






